Nasal COVID-19 vaccines help the body prepare for infection right where it starts – in your nose and throat
By Mayuresh Abhyankar, University of Virginia
Imagine inhaling just a few drops of liquid or mist to get protected from COVID-19. That is the idea behind nasal COVID-19 vaccines, and they have been getting a lot of attention recently as a spray or liquid. These nasal vaccines would be based on the same technology as normal vaccines given by injection. But as Mayuresh Abhyankar, a University of Virginia researcher who studies infectious diseases and works on nasal vaccines, explains, vaccinating someone right where the coronavirus is likely to start its attack comes with many immunological benefits.
1. What are nasal vaccines?
Nasal vaccines are administered, as the name suggests, through the nose. More accurately called intranasal vaccines, these vaccines are liquids that can be given as a spray or through a dropper or syringe. The most common nasal vaccine is FluMist, a nasal spray that uses inactivated flu virus to protect against influenza. An intranasal vaccine could be a weakened live virus similar to FluMist, a nucleic acid vaccine like mRNA coronavirus vaccines or a protein vaccine like Hepatitis B vaccines or the CorbeVax coronavirus vaccine.
Intranasal vaccines are best suited to protect against pathogens that enter through the nose, like the flu or the coronavirus. By mimicking the first step of natural exposure to an airborne pathogen, these vaccines help train a person’s immune system at the potential place of infection. Scientists have shown that the first immune response in the respiratory tract after a person is exposed to an airborne virus can influence how sick a person gets. So in theory, intranasal vaccines could provide better protection than vaccines given through a shot in the arm.
2. How does the coronavirus infect people?
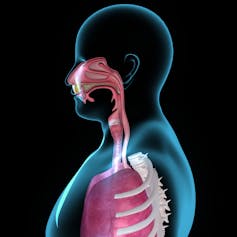
SARS-CoV-2, the virus that causes COVID-19, usually enters the body through the nose and lands on the mucus membrane at the back of the nasal passage and in the throat. The virus then enters the cells it touches, replicates and spreads.
Just underneath these cells of the mucus membrane are many types of immune cells that form what is called the mucosal immune system. Cells of the mucosal immune system are the first to identify invading coronavirus particles and start mounting a protective response.
In an unvaccinated individual, it takes about two weeks for these immune cells to build up a protective response after encountering the coronavirus. By that time, the virus can easily have infected other body parts, like the lungs, which can lead to severe disease.
Nasal vaccines follow a lot of the same steps. When you inhale a nasal vaccine, the particles land on the mucus membrane in your nasal cavity or the back of your throat, enter the cells in those places and trigger an immune response. This process teaches the body about the coronavirus and allows it to deal with any future real infections.
3. How are nasal and intramuscular vaccines different?
When you get a COVID-19 shot in your arm, the vaccine triggers a strong immune response in the cells near where you got the shot. It also causes your immune system to produce some coronavirus-specific antibodies and other immune cells in other locations throughout your body.
When the coronavirus begins infecting cells in a person’s respiratory tract, the immune cells nearby will start mounting a defense. Your body will also send anti-viral immune cells and antibodies from other locations to the site of infection. But by the time enough coronavirus-specific immune cells gather around the infection site to stop the virus from replicating, the virus has likely already begun to spread throughout the body, making it difficult for the immune system to keep up.
Nasal vaccines mimic the virus in order to prepare the immune system against a virus, just like any other vaccine. But importantly, they mimic the process of infection, too, and boost protective response within the mucosal immune system of the nose and throat. In simple terms, intranasal vaccines are like knowing there is going to be a break-in and putting your guards in the right location before the trouble even starts.
The science bears this idea out. In a head-to-head comparison, AstraZeneca’s COVID-19 vaccine provided greater protection in hamsters that were vaccinated intranasally compared to those vaccinated intramuscularly.
Nasal vaccination could also be used in concert with intramuscular immunization. In a recent study, my colleagues and I gave some mice both a nasal and intramuscular vaccine and exposed them to a lethal dose of SARS-CoV-2 – 100% of these mixed-vaccinated mice survived, compared to only 10% of the unvaccinated mice. We are now testing if this mixed approach is superior to just intranasal or just intramuscular approaches on their own.
Finally, intranasal vaccines are painless, noninvasive and do not require specialized training to use.
4. What are the risks of nasal vaccines?
Getting the dosage correct can be harder with nasal vaccines than a shot, especially with young children. If someone has a stuffy nose or sneezes out a part of the vaccine before it’s completely absorbed, this can result in a lower-than-desired dose.
There are some unique health risks too. All vaccines go through rigorous safety testing and clinical trials, but these processes are especially important for nasal vaccines due to the simple fact that the nose is close to the brain. In 2000, 27.7% of people who received an inactivated intranasal influenza vaccine in Switzerland developed transient facial paralysis – also known as Bell’s palsy. Later, researchers found that a bacterial toxin added to the vaccine to enhance the immune response was the culprit.
This is the only reported instance of neurological issues stemming from intranasal vaccines, but it is something to consider.
5. How long until intranasal COVID-19 vaccines are ready?
As of late May 2022, there are no approved COVID-19 intranasal vaccines for human use. There are currently seven in clinical trials, and three of them – manufactured by Beijing Wantai Biological Pharmacy, Bharat Biotech, and Codagenix and Serum Institute of India – are in phase-3 human trials.
In the coming months, the results of these trials will not only show how safe these promising new vaccines are, but also if they perform better than the vaccines in use today.![]()
Mayuresh Abhyankar, Associate Professor of Infectious Diseases and International Health, University of Virginia
This article is republished from The Conversation under a Creative Commons license. Read the original article.




